Hydrophobic Hydration and Interaction of Small Apolar Particles
We examine a temperature series of molecular dynamics simulations in order to study heat capacity effects associated with the hydrophobic hydration and interaction. For different water models we calculate the excess chemical potential for hydrophobic particles, as well as the solvation enthalpy and excess heat capacities. A 12 ps mpeg-movie (7.2 MByte) illustrates the rather dynamical hydrogen bond fluctuation in the hydration shell.
A spatial energie analysis allows a separation of the
solvation enthalpy into solute/solvent
and solvent/solvent part of the solvation enthalpy
and heat capacity. We find that the solvent/solvent contribution
to the excess heat capacity is dominating, being about
one order of magnitude larger than the solute/solvent part.
Hence the heat hydrophobic heat capacity effect has to be attributed
to the enlarged heat capacity of the water molecules in the
hydration shell.
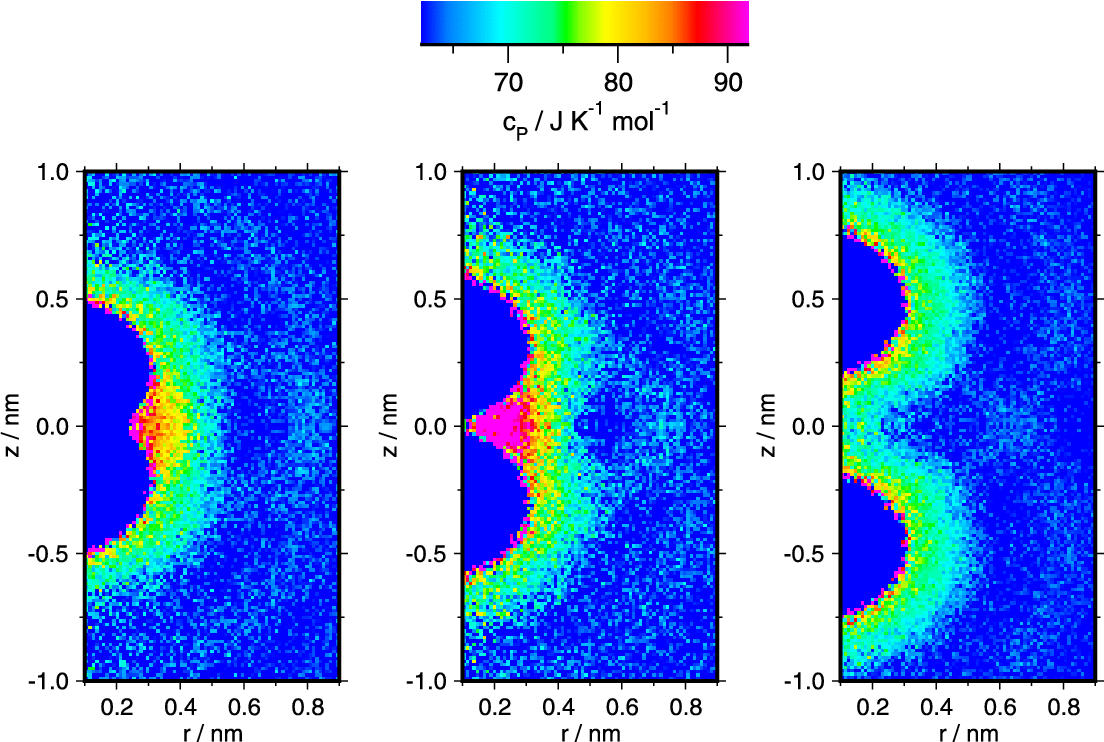
A detailed spatial analysis of the heat capacity of the water molecules around a pair of Xenon particles at different separations reveals that the even more enhanced heat capacity of the water located in the bisector plane between two adjecent Xenon atoms is responsible for the maximum of the heat capacity found for the desolvation barrier distance. The about 60 % enlarged heat capacity of water in the concave part of the joint Xenon-Xenon hydration shell is the result of a counterplay of strengthened hydrogen bonds and an enhanced breaking of hydrogen bonds with increasing temperature. Differences between the two models with respect to the heat capacity in the Xenon-Xenon contact state are attributed to the different water model bulk heat capacities, and to the different spatial extension of the structure effect introduced by the hydrophobic particles. Similarities are observed between the different states of water in the joint particle-particle hydration shell and the properties of stretched water.
References
- D. Paschek, A. Geiger , M. J. Herve, D. Suter: Adding Salt to an Aqueous Solution of t-Butanol: Is Hydrophobic Association Enhanced or Reduced? , J. Chem. Phys. 124, 154508 (2006). (PDF)
- P. E. Krouskop, J. D. Madura, D. Paschek, A. Krukau: Solubility of simple, non-polar compounds in TIP4P-Ew , J. Chem. Phys. 124, 016012 (2006). (PDF)
- D. Paschek: How the Liquid-Liquid Transition Affects Hydrophobic Hydration in Deeply Supercooled Water , Phys. Rev. Lett. 94 , 217802 (2005). (PDF)
- D. Paschek: Heat capacity effects associated with the hydrophobic hydration and interaction of simple solutes: A detailed structural and energetical analysis based on molecular dynamics simulations , J. Chem. Phys. 120, 10605-10617 (2004). (PDF)
- D. Paschek: Temperature Dependence of the Hydrophobic Hydration and Interaction of Simple Solutes: An E xamination of Five Popular Water Models, , J. Chem. Phys. 120, 6674-6690 (2004). (PDF)





